Ion-ion desolvation
This example demonstrates the effects of "desolvation" as modeled by reaction field terms in an implicit solvent setting. It illustrates that, while like charges do attract, the elimination of high dielectric material to form the charge-charge interface can introduce a substantial energetic barrier to association.
The scripts required to run this example are bundled in the tarball available for download here. This tarball should be uncompressed via:
$ gzip -dc ion-pmf.tar.gz | tar xvf -
|
Example 1. APBS input for ion-ion PMF
read
mol pqr complex.pqr # Two-ion complex
mol pqr mol1.pqr # Ion 1
mol pqr mol2.pqr # Ion 2
end
elec name comp_solv # Solvated complex
mg-manual
dime 97 33 33 # Grid dimensions
nlev 4 # Multigrid levels
glen 24 12 12 # Grid lengths
gcent 6 0 0 # Grid center on (6.0, 0.0, 0.0)
mol 1
lpbe # Linearized PB
bcfl mdh # Full multipole boundary condition
ion 1 0.000 2.0 # Zero ionic strength
ion -1 0.000 2.0 # Zero ionic strength
pdie 1.0 # Solute dielectric
sdie 78.54 # Solvent dielectric
chgm spl2 # Cubic spline charge discretization
srfm mol # Molecular surface
srad 1.4 # Solvent probe radius
swin 0.3 # Surface spline window
sdens 10.0 # Sphere density
temp 298.15 # Temperature
gamma 0.00072 # Apolar coefficient
write pot dx pot-solv # Inhomogeneous dielectric potential
calcenergy total # Total energy
calcforce no # No forces
end
elec name comp_vac
mg-manual
dime 97 33 33
nlev 4
glen 24 12 12
gcent 6 0 0
mol 1
lpbe
bcfl mdh
ion 1 0.000 2.0
ion -1 0.000 2.0
pdie 1.0
sdie 1.0
chgm spl2
srfm mol
srad 1.4
swin 0.3
sdens 10.0
temp 298.15
gamma 0.00072
calcenergy total
calcforce no
write pot dx pot-vac
end
elec name ion1_solv
mg-manual
dime 97 33 33
nlev 4
glen 24 12 12
gcent 6 0 0
mol 2
lpbe
bcfl mdh
ion 1 0.000 2.0
ion -1 0.000 2.0
pdie 1.0
sdie 78.54
chgm spl2
srfm mol
srad 1.4
swin 0.3
sdens 10.0
temp 298.15
gamma 0.00072
calcenergy total
calcforce no
end
elec name ion1_vac
mg-manual
dime 97 33 33
nlev 4
glen 24 12 12
gcent 6 0 0
mol 2
lpbe
bcfl mdh
ion 1 0.000 2.0
ion -1 0.000 2.0
pdie 1.0
sdie 1.0
chgm spl2
srfm mol
srad 1.4
swin 0.3
sdens 10.0
temp 298.15
gamma 0.00072
calcenergy total
calcforce no
end
elec name ion2_solv
mg-manual
dime 97 33 33
nlev 4
glen 24 12 12
gcent 6 0 0
mol 3
lpbe
bcfl mdh
ion 1 0.000 2.0
ion -1 0.000 2.0
pdie 1.0
sdie 78.54
chgm spl2
srfm mol
srad 1.4
swin 0.3
sdens 10.0
temp 298.15
gamma 0.00072
calcenergy total
calcforce no
end
elec name ion2_vac
mg-manual
dime 97 33 33
nlev 4
glen 24 12 12
gcent 6 0 0
mol 3
lpbe
bcfl mdh
ion 1 0.000 2.0
ion -1 0.000 2.0
pdie 1.0
sdie 1.0
chgm spl2
srfm mol
srad 1.4
swin 0.3
sdens 10.0
temp 298.15
gamma 0.00072
calcenergy total
calcforce no
end
print energy comp_solv - comp_vac - ion1_solv + ion1_vac - ion2_solv + ion2_vac end
quit
|
The series of APBS calculations can be started by typing:
$ bash runme.sh
|
the energy of interaction,
the Coulomb energy for the homogeneous system (solvent dielectric),
and the relative positions of the ions with respect to a solvent-sized probe.
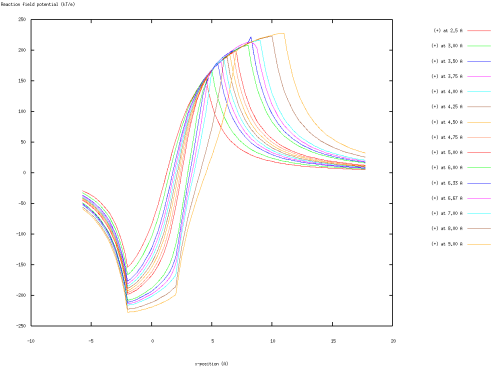
This script also calculates the reaction field potential (the potential for the fully-solvated system minus the potential for the vacuum system) along the x-axis. These potentials can be visualized via Gnuplot by typing:
$ gnuplot -persist rxnfield.gnuplot
|