Origins: Analyzing the Origin of Homochirality in Life (Math 131C)
With a glass-tube flask, a few chemicals, and a spark of electricity, Stanley Miller in 1953 performed an experiment that was credited for turning lifeless soup into the building blocks for life. Renewing the debate on the origin of life, the Miller-Urey experiment was hailed by some as evidence that the first life on earth could have originated purely by stochastic processes. Indeed, two percent of the resulting chemicals were amino acids, which are the building blocks of proteins [1].
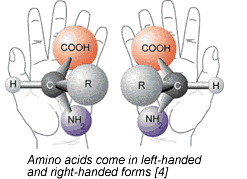 However, there was a slight complication to Miller's results. Miller's brew of amino acids was a racemic mixture : approximately 50% of amino acids were in the laevorotatory form (left-handed orientation), and 50% were in the dextrorotatory form (right-handed orientation) [2]. It is a well-known fact that virtually all of observable life is composed of 100% L-amino acids. This phenomenon, known as homochirality (or biochirality), was first discovered by Louis Pasteur and has puzzled scientists for many years [3]. Could this feature of homochirality in life have arisen by blind chance? Perhaps statistics can help to find the answer.
However, there was a slight complication to Miller's results. Miller's brew of amino acids was a racemic mixture : approximately 50% of amino acids were in the laevorotatory form (left-handed orientation), and 50% were in the dextrorotatory form (right-handed orientation) [2]. It is a well-known fact that virtually all of observable life is composed of 100% L-amino acids. This phenomenon, known as homochirality (or biochirality), was first discovered by Louis Pasteur and has puzzled scientists for many years [3]. Could this feature of homochirality in life have arisen by blind chance? Perhaps statistics can help to find the answer.
Homochirality and Chance
In constructing a simple model of the amino acid synthesis, let us make the following assumption: left and right-handed amino acids were evenly distributed in early earth. Let us also assume that at least 500 amino acids are necessary to form one functioning polymer.
Thus the probability that a 500-long strand of amino acids is 100% left-handed is simply 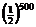 . This type of statistic, reported in other books [5], simply comes from the multiplication rule. Embedded is the assumption that the selection of amino acids is independent and identically distributed. Some may question this assumption (namely the feature of independence) and claim that natural selection may have been used to get a 100% left-handed strand. However, by definition, natural selection cannot form prior to the formation of life, since there is no life to select [3].
. This type of statistic, reported in other books [5], simply comes from the multiplication rule. Embedded is the assumption that the selection of amino acids is independent and identically distributed. Some may question this assumption (namely the feature of independence) and claim that natural selection may have been used to get a 100% left-handed strand. However, by definition, natural selection cannot form prior to the formation of life, since there is no life to select [3].
Let us imagine that the synthesis of amino acids was robust enough to allow as many as 10 out of the 500 amino acids to be right-handed. Then we could set up a basic statistical hypothesis test. The null hypothesis would be that blind chance was used in the formation of the amino acid strand. In other words, p0 = 50% , since blind chance does not differentiate between L and D amino acids. The alternative hypothesis would be that p > p0, where p is the actual probability. Let W be a random variable having a distribution Bin(n, p0 ) , where n = 500 . Let 0.001 be our significance level (a). Then:
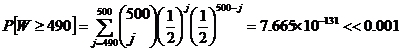
This result shows that we can reject our null hypothesis in favor of our alternative hypothesis ( p > p0). If we did a two-sided test with alternative hypothesis p ≠ p0, the statistic above would double. Even with the doubled statistic, the test indicates that blind chance is woefully inadequate to select 490 or more amino acids (of the same orientation) out of 500.
Furthermore, we could estimate a 99% confidence interval for p by using the Laplace-DeMoivre theorem [6]. Let x0 = 2.575 , n = 500 , and Sn = 490 (since we allow 10 right-handed amino acids). Then by the theorem:
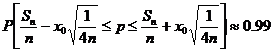
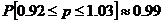
Notice that the upper bound for p exceeds 1! This occurs for two reasons: we are analyzing the extreme tail of the binomial distribution, and there is an approximation made when isolating p . Therefore, in order to maintain the confidence interval, let us correct the error and shift both bounds left by 0.03. The result tells us that 99% of the time, our confidence interval [0.89, 1] will bound p .
From the relatively simplistic statistics above, we can rule out blind chance as a cause for homochirality. By utilizing either intuition or mathematical rigor, the scientific community has already come to the same conclusion as we have. Even Stanley Miller concedes, “There is no way in my opinion that you are going to sort out the D and L amino acids in separate pools" [2].
Instead, scientists have tried to find natural causes for an initial enantiomeric excess (a greater abundance of one orientation of amino acids). Once an excess can be explained, then homochirality may be feasible as well, they argue. Listed are the more visible theories:
- There may be a natural bias in selecting left-handed amino acids that comes various sources, like a difference in potential energy. This bias can be amplified by reactions like the Soai reaction [7].
- Early life forms may have been originally racemic, and the feature of homochirality may have evolved later [2].
- Some places of outer space may have generated left-handed amino acids more frequently than right-handed amino acids. A collection of predominantly left-handed amino acids could have been transported to earth via meteorites (like the Murchison meteorite) [8].
Although the first theory may account for some small excesses, the amplification of these excesses seems to occur only in very specific conditions. Furthermore, some assumptions are embedded in the first theory. One such assumption, presented by Blackmond, is that “homochiral and heterochiral species must have different activities as catalysts” [7]. The second theory is interesting as well, but the proposed racemic life seems to be purely theoretical and conjectural. In any case, we will focus on the third theory because there are some tangible results associated with this theory.
Homochirality from Space?
The Murchison meteorite, which was found near Melbourne, Australia in 1969, has done much to bolster the theory that homochirality may have been shipped to earth from other parts of the solar system through meteorites.
Some scientists who have analyzed the Murchison meteorite claim that enantiomeric excesses exist in several of the amino acids that were found in the meteorite. One such set of Murchison data is shown in Table 1 [8]. The excess percentage shown represents the ratio between the number of L-amino acids and the number of D-amino acids. While the majority of tests were done on Chirasil, four other amino acids were tested as well. One assumption that we make is that the variances (or error present) during the chromatography of each amino acid are equivalent. This assumption is valid, since a correctional factor was used on the data of each amino acid.
The essential question is whether or not the enantiomeric excesses found in the Murchison meteorite can account for homochirality in life. We will try to answer this question by first trying to find a common mean.
Let me note that the approximate abundance of L-amino acids (per arbitrary unit) is an estimate that is calculated by multiplying the percentage of L-amino acids by a factor of 10^6. This is a standard factor, since other papers give amino acid abundances on the range of 10^6 to 10^7 as well [9].
Group (or Sample) |
Amino Acid |
Enantiomeric excess (%) (corrected) |
Approx. abundance of L-amino acids (per unit, multiplied by 10^6). |
1 |
Chirasil-L-Val, acid-hydrolyzed (2S, 3S) |
7.6 |
538000 |
1 |
Chirasil-L-Val, unhydrolyzed (2S, 3S) |
7.6 |
538000 |
1 |
Chirasil-D-Val, unhydrolyzed (2S, 3S) |
5.8 |
529000 |
1 |
Chirasil-L-Val, acid-hydrolyzed (2S, 3R) |
7.6 |
538000 |
1 |
Chirasil-L-Val, unhydrolyzed (2S, 3R) |
9.2 |
546000 |
1 |
Chirasil-D-Val, unhydrolyzed (2S, 3R) |
10.4 |
552000 |
2 |
Isovaline |
8.4 |
542000 |
2 |
a-Methylnorvaline |
2.8 |
514000 |
3 |
a-n-butyric acid |
0.4 |
502000 |
3 |
Norvaline |
0.4 |
502000 |
Table 1. Enantiomeric excesses for different amino acids in the Murchison meteorite [8]
Let us break up the data into 3 different groups. The first group consists of the data on Chirasil. The second group consists of the other amino acids that have significant enantiomeric excesses (Isovaline and a-Methylnorvaline). The third group consists of the amino acids which are essentially racemic (a-n-butyric acid and Norvaline).
We would like to know whether or not there is a common mean between the groups that are presented in Table 1. We could use the general linear model [6] to characterize this data:
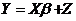
where
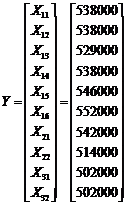 ,
, 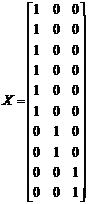 ,
, 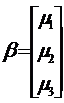 ,
, 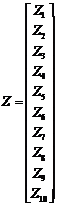
Notice that the Y-vector is composed of the observed values in Table 1. The design matrix X is constructed from the sample groupings, and the ß-vector contains the expectations of each of the samples. The Z-vector is composed of independent normal random variables with parameters (0, s^2 ).
From the general linear model, we can set up a null hypothesis, H0 : µ1 = µ2 = µ3, and an alternative hypothesis that claims that the expectations are not all equivalent. Let a = 0.01.
We can compute the following statistic and compare it to the F-distribution with parameters (2, 7). The first parameter equals the number of groups (3) minus 1, and the second parameter equals the number of total entries (10) minus the number of groups (3).
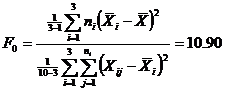
The statistic above was computed on a spreadsheet in Microsoft Excel. If we look at a table for the F-distribution [10], we find that P[ F(2, 7) = 10.90 ] < a = 0.01 . Therefore, we can reject the null hypothesis that the means are equal. Intuitively, this result makes sense, because a sample that is racemic and a sample that has perceived enantiomeric excesses should not have the same mean.
Let us perform the statistical test again and this time let us ignore the last group in the table (the racemic group). Our new null hypothesis is H0 : µ1 = µ2. Using the same method as above, we get the statistic F0 ' = 1.89. After comparing the new statistic with the F(1, 6) distribution, we can readily accept the new null hypothesis.
Now, since µ1 = µ2, we can treat the two groups (or samples) as one sample [11]. To find the probable amounts for the enantiomeric excesses, it is useful to construct a confidence interval of µ based on the data for the two groups. In this model, we continue to ignore the last group, because it is already racemic. We can use the T distribution to get the confidence interval [6].
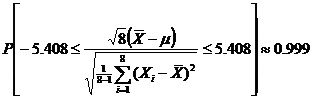
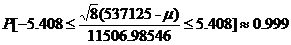
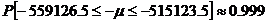
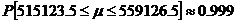
So the 99.9% confidence interval for µ is [515123.5, 55916.5]. Knowledge of this confidence interval for the mean also gives knowledge of the bounds of the possible L-amino acid percentages as well (since we used the same arbitrary unit to analyze each amino acid). After dividing by the factor of 10^6, we discover that the percentage of L-amino acids is bounded by [51.5%, 55.9%] with 99.9% accuracy.
Let us revisit the first statistical test on homochirality and chance, and let us now use p = 55.9% instead of the original p = 50% . We use p = 55.9% to simulate the Murchison meteorite's slight bias towards the L-amino acids.
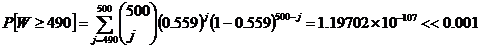
With p = 55.9% , there is a slightly more probable chance of getting 490 out of 500 L-handed amino acids. However, this statistic is still very, very low, and we must reject the null hypothesis that chance, with slight bias, could have produced homochirality.
Dealing With Small Probabilities
In the previous sections, we have dealt with probabilities of extremely low magnitudes. A significant question is whether or not an extremely small probability virtually precludes an event from ever happening. Practically, statisticians disregard events of small probability all the time (as long as the probability is below the arbitrary a). However, in theory, some statisticians are reluctant to conclude that an event with an extremely small probability will not happen [12].
One may employ the following logic to accept the idea that stochastic processes could have brought about homochirality, regardless of its improbability:
Theoretically, homochirality arising through stochastic processes should be improbable. However, we observe homochirality in life now. Since homochirality has happened, we should probably revise that theoretical probability (there may have been some lurking variables) or we may just consider ourselves as being extremely lucky. Besides, given an almost infinite amount of time, any possible thing can happen. In fact, homochirality is bound to happen, if you model the selection of amino acids as a Markov chain with a reflexive barrier on one end. The only thing that is necessary is a transition to the next state. The probability associated with that transition doesn't matter, so long as it is a fixed, finite mass. So inevitably, chance would produce a strand of L-amino acids of length 500.
However, there is a bound on the probabilistic resources that chance can use to try to arrive at homochirality. William Dembski computes such a bound by using the following logic [13]:
- There is a maximum of 10^80 elementary particles in the universe.
- Transitions from one physical state to another cannot occur faster than 10^45 times per second.
- The universe is much younger than 10^25 seconds.
Dembski argues that the total number of specified events throughout the universe's history cannot be greater than 10^80 * 10^45 *10^25 = 10^150 . This is the upper bound for the probabilistic resources that are available to chance.
Let me similarly construct an upper-bound logical proof that relates to homochirality:
- Keeping in mind that there are 10^80 particles, I estimate there to be 10^50 sets of 500 amino acids.
- I estimate that the synthesis of 500 amino acids can happen no faster than 10^25 times per second.
- Let us suppose that amino acid synthesis can happen every second that the universe has ever existed (upper-bounded to be 10^25 ).
From the above figures, the probabilistic resource available to construct a homochiral strand of 500 amino acids is 10^100 . We can think of this figure as the maximum number of (independent) trials that can happen. Earlier, we computed the probability that a strand can have 490 or more L-handed amino acids given a slight enantiomeric excess: it was 1.19 * 10^-107 . So we can model the amount of homochiral strands that can stochastically occur in the universe's history as a binomial random variable with parameters (10^100 , 1.19 * 10^-107 ). From these parameters, we can see that the expected number of homochiral strands is 1.19 * 10^-7 , a figure that is not even close to 1.
All of this analysis leads to a contradiction. One out of the two propositions below must be wrong:
- Obtaining homochirality through stochastic processes is virtually impossible.
- The feature of homochirality is observed today and is abundant in life.
Since proposition 2 has been empirically proven and cannot be wrong, proposition 1 must be wrong. Either homochirality is not virtually impossible, or homochirality did not come from undirected, stochastic processes (or we are extremely lucky, but we can reject this notion because we have already curbed our luck through the statistical analysis above). As we have seen in the analysis above, the statistical results convincingly show that chance chemistry cannot produce homochirality. So we are left to conclude that homochirality came about by some directed, non-stochastic mechanism.
References :
[1] “Miller-Urey Experiment”. Wikipedia. <http://en.wikipedia.org/wiki/Stanley_Miller>.
[2] Interview with Stanley Miller, by S. Henahan. 1996. <http://www.accessexcellence.org/WN/NM/miller.html>.
[3] J. F. Coppedge. Evolution: Possible or Impossible? Santa Clarita, CA: Prob. Research in Mol. Biology, 2002.
[4] Origin of Life: the Chirality Problem . (Diagram). <http://www.answersingenesis.org/docs/3992.asp>.
[5] S. C. Meyer. The Explanatory Power of Design: DNA and the Origin of Information . In Mere Creation . Ed. William Dembski. Downer's Grove, Ill: Intervarsity Press, 1998. 125.
[6] H. G. Tucker. Some Theory and Practice of Statistics. (Internet Textbook). <http://math.uci.edu/~htucker/StatisticsBook/>.
[7] D. G. Blackmond. Asymmetric autocatalysis and its implications for the origin of homochirality. 2004. <http://www.pnas.org/cgi/reprint/101/16/5732.pdf>.
[8] J. R. Cronin, S. Pizzarello. Enantiomeric Excesses in Meteoritic Amino Acids . Science . Vol. 275. 1997. 951-955.
[9] J. R. Cronin, S. Pizzarello. Scientific Correspondence: Alanine enantiometers in the Murchison meteorite . Nature . Vol 394. 1998. 236.
[10] D. S. Moore , G. P. McCabe. Introduction to the Practice of Statistics . 4th edition. New York: W. H. Freeman, 2003.
[11] Email conversation with Professor ____, June 11, 2004.
[12] Conversation with Professor ____, June 10, 2004.
[13] W. Dembski. The Design Inference: Eliminating Chance Through Small Probabilities . Cambridge: Cambridge University Press. 1998. 209-210.
Note: In the propositions above, there should be an additional implied default proposition that says that homochirality was obtained through stochastic processes. With this implied proposition in mind, the last paragraph makes more sense.